The wide interest towards molecular machines is witnessed by the Nobel Prize recently assigned to Profs. Feringa, Sauvage and Stoddart (2016). An important role in the frame of molecular machines has been played by catenanes and rotaxanes in which the relative position of the components is controlled by the presence of switchable functions. The distinctive feature of these systems is the ability to perform back and forth motions,
i.e. a series of two or more roto-translations during which the machine varies from an initial state A to one or more intermediate states B’, B”,…, and eventually reverts back to the initial state A. The transition between states A and B usually requires the sequential addition of a fuel and an antifuel, such as an acid and a base, a reductant and an oxidant, irradiation with light of different wavelengths and the like. Conversely, systems in which only one stimulus is required to guarantee the operation of a back and forth motion are rare. We recently realized one of these systems, which is based on the decarboxylation reaction of a series of activated carboxylic acids. In a first step the acid donates a proton to an acid-base operated molecular switch that passes from state A to state B. The subsequent decarboxylation of the resulting carboxylate produces a carbanion that is a very strong base able to take back the proton from the protonated switch to restore the state A from state B. Our effort is now devoted to the application of our chemical fuel to other acid-base operated molecular machines.
Related representative publications:
C. Biagini, S. Albano, R. Caruso, L. Mandolini, J. A. Berrocal, S. Di Stefano, Chemical Science, 2018, 9, 181 – 188.

Moderate variations in the fuel structure cause large changes in the rate of the back and forth motions experienced by a chemically fuelled catenane-based switch.
J. A. Berrocal, C. Biagini, L. Mandolini, S. Di Stefano, Angewandte Chemie International Edition 2016, 55, 6997–7001.
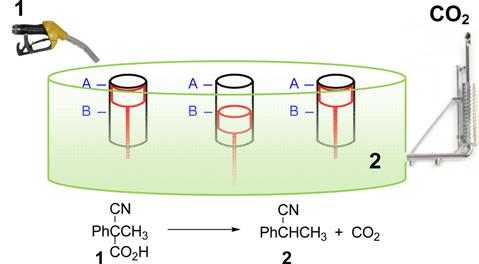
Carboxylic acid 1 is described as a convenient fuel for the operation of a molecular switch that move under the influence of protonation-deprotonation steps. The cyclic motions of the switch take place at the sole expenses of the chemical energy supplied by the decarboxylation of 1, without recourse to additional stimuli.