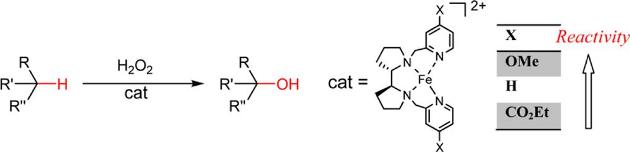
Non-heme iron and manganese complexes are emerging as powerful and versatile catalysts in several oxidative transformations. Remarkable advantages associated with such catalysts are the ease of their synthesis and the related use of environmentally friendly compounds such as H2O2 as terminal oxidants. The most investigated non-heme iron- and manganese catalytic complexes are based on aminopyridine ligands, although a number of imine-based ligands have been recently considered. We have lately used a supramolecular approach in the field of aminopyridine-based iron and manganese complexes to catalyze the selective oxidation of particular methylenic positions of long, linear, primary amines. The binding event responsible of the recognition between the catalyst and the substrate allows for an unprecedented selectivity. Concerning the imine-based catalysts, we introduced an imine-based iron complex, easily prepared in situ from cheap and commercially available starting materials, which was demonstrated to be able to catalyze the oxidation of aliphatic and aromatic C−H bonds. We are currently investigating on the possible expansion of the synthetic scope of our supramolecular aminopyridine-based complex and on the mechanism of action of the imine-based iron complex.
Related representative publications:
G. Olivo, G. Farinelli, A. Barbieri, O. Lanzalunga, S. Di Stefano, M. Costas, Angewandte Chemie International Edition 2017, ASAP DOI: 10.1002/anie.201709280.
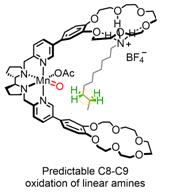
Supramolecular recognition of protonated primary amines on 18-crown-6 receptors exposes specific, remote methylenes to the Mn active site. Linear alkyl chains can thus be selectively oxidized on C8 and C9 positions with H2O2, overriding the intrinsic reactivity of C-H bonds.
G. Capocasa, G. Olivo, A. Barbieri, O. Lanzalunga, S. Di Stefano, Catalysis Science & Technology 2017, Advance Article DOI: 10.1039/C7CY01895A.
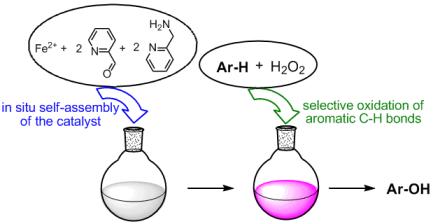
An imine-based catalyst easily obtained by self assembly of cheap and commercially available starting materials, selectively catalyzes the hydroxylation of aromatic compounds.
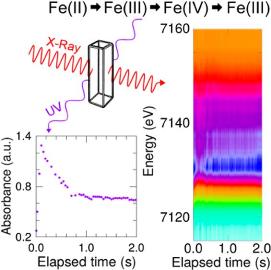
G. Olivo, A. Barbieri, V. Dantignana, F. Sessa, V. Migliorati, M. Monte, S. Pascarelli, T. Narayanan, O. Lanzalunga, S. Di Stefano, P. D’Angelo, Journal of Physical Chemistry Letters 2017, 8, 2958−2963.
G. Olivo, O. Lanzalunga, S. Di Stefano, Advanced Synthesis & Catalysis, 2016, 358, 843−863.
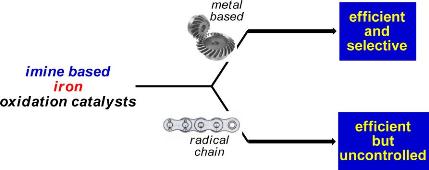
G. Olivo, M. Nardi, D. Vìdal-Sanchez, A. Barbieri, A. Lapi, L. Gómez, O. Lanzalunga, M. Costas, S. Di Stefano, Inorganic Chemistry 2015, 54, 10141–10152.
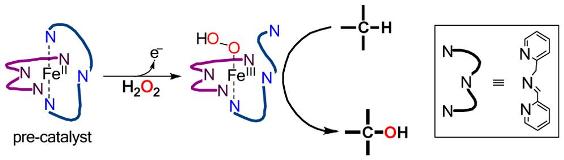
G. Olivo, O. Lanzalunga, L. Mandolini, S. Di Stefano, Journal of Organic Chemistry 2013, 78, 11508–11512.